All matter is made of up particles, these particles behave in different ways whether they are solid, liquid or gas.
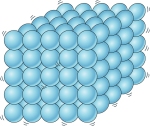
Solids
Particles in solids are held together very closely.
This makes them very strong and difficult to break apart. Solids can also hold their own shape
The particles don’t move around very much but simply vibrate in their spot.
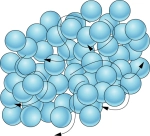
Liquids
Particles in liquids are quite close to each other, however they can move past each other very easily.
This makes liquids very easy to break apart and why they cannot hold their own shape, but instead take the shape of their container
The particles in liquids move around quite a bit, bumping gently past each other.
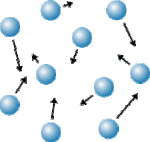
Gases
Particles in gases move around very quickly with a lot of space between them, this means you can compress them very easily.
Gases don’t just take the shape of their container they fill the space of the container that they are in
Change of state
Solids, liquids and gases can of course change between each other simply by heating or cooling them. The processes involved in changing each states are
| Starting state | Finishing state | Process |
|---|---|---|
| solid | liquid | melting |
| solid | gas | sublimation |
| liquid | solid | freezing |
| liquid | gas | evaporation |
| gas | liquid | condensation |
You must be logged in to post a comment.